【Product Name】
Novel Coronavirus (COVID-19) Nucleic Acid Detection Kit
(Real-time RT-PCR Method)
【Components of the Diagnostic Kit】
| No. | Reagent Name | Spec. & Qty. | | Color of screw cap |
| | 50T | 200T | |
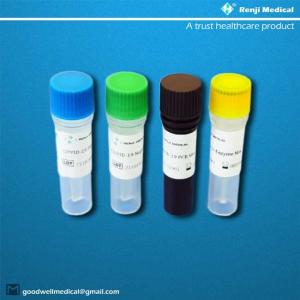
| 1 | COVID-19 PCR Mix | 750µl x 1 tube | 750µl x 4 tube | Brown |
| 2 | COVID-19 Enzyme Mix | 250µl x 1 tube | 250µl x 4 tube | Yellow |
| 3 | COVID-19 Negative Control | 100µl x 1 tube | 100µl x 4 tube | Green |
| 4 | COVID-19 Positive Control | 100µl x1tube | 100µl x4tube | Blue |
| 5 | Instructions | 1 serving | 1 serving | |
Note:
1. Do not mix or exchange components from different kit lots.
2. The COVID-19 Negative Control is sterile nuclease-free water,
and the COVID-19
Positive Control is transcribed RNA invitro which contains target
genes ORF1ab,
N gene, and Internal Control gene.
Renji Coronavirus Nucleic Acid Detection Kit is a real-time RT-PCR
in vitro
diagnostic test intended for the qualitative detection of nucleic
acid from
SARS-CoV-2 in human oropharyngeal swab and nasopharyngeal swab
specimens
collected by a healthcare provider (HCP), and anterior nasal swab
specimens
collected by an HCP or self-collected under the supervision of an
HCP by any
individual, including individuals without symptoms or other reasons
to suspect
COVID-19. This test is also for use with saliva samples collected
using the Renji
Saliva Collection Kit either by an HCP or self-collected under the
supervision of
an HCP in a healthcare setting from individuals suspected of
COVID-19.
【Intended Use】
Renji Coronavirus Nucleic Acid Detection Kit is intended for use by
qualified clinical
laboratory personnel specifically instructed and trained in the
techniques of
real-time PCR and in vitro diagnostic procedures. The Coronavirus
Nucleic Acid
Detection Kit is only for use under the Food and Drug
Administration’s Emergency
Use Authorization.
2019-nCoV detection kit is used for in vitro qualitative detection
of the ORF1ab and N
genes of SARS-CoV-2 RNA in nasopharyngeal swabs and sputum
specimens of
suspected pneumonia cases, suspected cluster cases infected by
novel coronavirus, and
other patients requiring diagnosis or differential diagnosis of the
novel coronavirus
infection. The function of the assay is for the diagnosis or aid
for diagnosis of COVID-19.
For the definitions of ‘suspected cases’ and ‘suspected clustered
cases’, refer to the
documents (current version) such as ‘Diagnosis and Treatment Plan
for Pneumonia
Infected by Novel Coronavirus’ and ‘Monitoring Plan for Pneumonia
Cases Infected
by Novel Coronavirus’issued by CDC.
This product is only used in the pneumonia epidemic of SARS-CoV-2
infection since
December 2019. The auxiliary diagnosis of related cases and the in
vitro diagnostic
epidemic reserve of this epidemic cannot be used as a conventional
in vitro diagnostic
reagent for clinical application. In use, the relevant requirements
of the ‘Pneumonitis
Diagnosis and Treatment Scheme for Novel Coronavirus Infection’ and
‘Pneumonitis
Prevention and Control Scheme for Novel Coronavirus
Infection’should be complied.
To carry out nucleic acid detection of novel coronavirus,
experimenters should conduct
biosafety procedures and conform to the requirements of ‘Technical
Guidelines for
Laboratory Monitoring of novel coronavirus infected pneumonia’ by
CDC.
This kit is used for the detection of COVID-19(ORF1ab/N Gene)
Nucleic Acid and
assisted diagnosis and epidemiological surveillance of COVID-19.
【Features】
››› Efficient: Pooling of multiple samples increases throughput
and decreases costs
››› Low cost, easy, convenient kit!
››› Perfect for remote or mobile testing!
››› FAST: Complete results in 40-90 mins, from start to finish!
【Package Specification】
50 tests/kit & 200 tests/kit
【Test Principle】
The kit adopts the principle of real-time fluorescent quantitative
PCR technology,
designs specific primers and probes for COVID-19(ORF1ab/N gene) ,
and combines them with real-time fluorescent quantitative PCR
instrument to
detect the nucleic acid of COVID-19 virus. So as to realize the
qualitative detection
of COVID-19 virus nucleic acid.
In addition, the PCR detection system uses the positive internal
control,
which monitors the presence of PCR inhibitors in test specimens by
detecting
whether the internal control signal is normal,to avoid a false
negative result.
The assay is based on the real-time polymerase chain reaction (PCR)
technology
and is composed of a ready to use optimized mixture with target
specific primers
and probes for the detection of SARS-CoV-2. Probes consist of a
fluorophore
covalently attached to the 5’-end of the oligonucleotide probe and
a quencher at
the 3’-end. The quencher molecule quenches the fluorescence emitted
by the
fluorophore when excited by the cycler’s light source. As long as
the fluorophore
and the quencher are in proximity, quenching inhibits any
fluorescence signals.
Degradation of the probe releases the fluorophore from it and
breaks the proximity
to For Emergency Use Only Instructions For Use rev.1.2 2 the
quencher, thus relieving
the quenching effect and allowing fluorescence of the fluorophore.
Hence, fluorescence
detected in the quantitative PCR thermal cycler is directly
proportional to the
fluorophore released and the amount of DNA template present in the
PCR.
【Applicable Instrument】
The diagnostic kit is applicable to MA-6000,ABI series, Bio-Rad
series, Roche
LightCycler R480, Cepheid SmartCycler, Rotor-Gene series and other
multi-channel
real-time quantitative PCR instruments.
【Specimen Requirements】
1. Sample types:Upper respiratory tract specimens(including throat
swabs,
nasal swabs, nasopharyngeal extracts deep cough sputum), lower
respiratory tract
specimens(including respiratory tract extracts, bronchial lavage
fluid alveolar lavage
fluid, lung tissue biopsy specimens) , Tissue culture and other
samples.
2.Storage conditions:The collected specimens should be submitted
for inspection
in a timely manner, and the specimens should be stored at 4℃ within
24 hours.
It is best to store at-70℃ for more than 24 hours, and avoid
repeated freeze-thaw
cycles.
【Storage and Stability】
1. The diagnostic kit should be stored in a sealed pouch
at-20±5℃.The expiry date
is 12 months.
2. Please refer to the date of manufacture and expiry date on the
outer package.
3. The reagent keep valid and stable within the expiry date if not
used. The kit should
not be frozen and more than 5 times.
【Test Method】
1.Reagent Preparation (performed at “reagent preparation region”)
1.1 Takeout each component from the diagnostic kit and place them
at room temperature.
Allow the reagents to equilibrate at room temperature, then vortex
each of them
respectively for later use.
1.2 According to the quantity of test specimens, COVID-19 Positive
Control and
COVID-19 Negative Control,pipette appropriate quantity of COVID-19
PCR Mix
and COVID-19 Enzyme Mix (COVID-19 PCR Mix 15 µl/test+COVID-19
Enzyme
Mix 5 µl /test) mix them thoroughly to make a PCR-Master mix
centrifuge it
instantaneously for later use.
| Reagent name | 1 Sample | 10 Samples | 25 Samples | 50 Samples | 100 Samples | 200 Samples |
| COVID-19PCR Mix(µl) | 15 | 150 | 375 | 750 | 1500 | 3000 |
COVID-19 Enzyme Mix(µl) | 5 | 50 | 125 | 250 | 500 | 1000 |
PCR-Mastermix
| 20 | 200 | 500 | 1000 | 2000 | 4000 |
| Note:The above configuration is just you’re your reference and to
ensure enough volume of the PCR-Mastermix, more volume of the
actual pipetting may be required. |
1.3 Transfer the above-prepared reagents to the "specimen
processing region" for
later use.
2.Processing and loading of specimens (performed at "specimen
processing region")
2.1 This diagnostic kit does not include Viral RNA&DNA
Extraction Kit. It is
recommended to use Viral RNA&DNA Extraction Kit produced by
Changsha Renji
Medical Equipment Co, Ltd. to extract viral RNA. The specific
operation is in
accordance with its instructions.
2.2 Add 20 µl PCR-Mastermix into PCR reaction tube with 5µl above
processed sample,
COVID-19 Positive Control and COVID-19 Negative Control and cap the
tube. Carry
out fluorescence quantitative PCR detection on fluorescence PCR
instrument.
3.PCR Amplification (performed at "nucleic acid amplification
area")
3.1 Place PCR reaction tubes into the specimen wells of the
amplification equipment
Setup COVID-19 Positive Control COVID-19 Negative Control and
specimens to be
tested in the corresponding sequence and input specimen name.
3.2 Set cycle parameters according to the following table for PCR
amplification.
| Steps | Temperature | Time | Cycles |
| 1 | 50°C | 10min | 1 |
| 2 | 95°C | 3min | 1 |
| 3 | 95°C | 10s | 40 |
| 55°C | 30s |
Note:
1) The fluorescence collection is set at "Step3:55°C, 30s".
Selection of detection
channels:FAM, HEX and Cy5, where FAM channel is ORF1ab gene and HEX
channel is N gene. Cy5 channel is the internal control gene, and
the reaction system
s set to 25 µl.
2) ABI series fluorescent PCR instruments do not select ROX
calibration and select
None for the quenching group.
【Positive Judgement Value and Reference Interval】
1. Condition Setting for Result Analysis
The adjustment principle of Baseline and Threshold is generally
based on the results
of the automatic analysis of the instrument. When the overall slope
of the curve
appears, the Start, End,and Threshold values of the Baseline can be
adjusted
according to the image. Usually, the user can adjust it according
to the actual
situation. The Start value can be set to 3-15, and the End value
can be set to 5-20.
Adjust the amplification curve of the negative control to make it
straight or below
the thresh hold line.
2. The Validity of The Kit
2.1 The COVID-19 Positive Control:FAM, HEX and Cy 5 channels have typical
S-type amplification curves and Cts≤32.
2.2 The COVID-19 Negative Control:FAM, HEX and Cy 5 channels have no
Ct or Ct>38.
2.3 Note:The above conditions must be met at the same time, otherwise this
experiment is invalid and needs to be repeated.
3.The Positive Judgment Value
Through the study of reference values, it was determined that the
Ct reference value
of the target gene and the internal control gene detected by this
kit was both 38.
【QUALITY CONTROL】
The kit product provides negative control, positive control, and
internal control to
monitor the reliability of the results for the entire batch of
specimens from sample
extraction to PCR amplification. All test controls should be
examined prior to
interpretation of patient results. Positive control, negative
control and IC in positive
and negative control should meet the requirements listed in the
below table to ensure
valid results. If the controls are not valid, the patient results cannot be
interpreted.
Result Interpretation of Test Controls for 60 µL reaction:
Result Interpretation of Test Controls for 30 µL reaction:
Result Interpretation of Test Controls for 15 µL reaction:
1) Negative Control: both ORF1ab and N of SARS-CoV-2 must be not
detected, and
the Ct value of internal control should be ≤ 40.
2) Positive Control: both ORF1ab and N of SARS-CoV-2 must be detected, and their
Ct values should fall within the ranges described in the above tables, the Ct value of
internal control does not have to be ≤40 for positive control.
【The Sample Result Judgment】
1.If the test sample detects atypical S-type amplification curve in
the FAM, HEX
and Cy 5 channels and the Ct is≤38, the sample can be judged to be
COVID-19
positive.
2.If the test sample has no Ct or Ct>38 in the FAM and HEX
channels, and there
is atypical S-type amplification curve in the Internal Control
channel(Cy 5) , Cts≤38,
the sample can be judged to be COVID-19 negative.
3.If the test sample only has a Ct values 38 in a single channel of
the FAM or HEX
channel, and there is atypical S-type amplification curve in the
Internal Control
channel(Cy 5) , Ct≤38, the results need to retest. If the results
repeated are
consistent the sample can be judged as COVID-19 positive, the
results repeated
are negative except for the typical S-type amplification curve of
the Internal Control
channel(Cy 5) , Ct≤38, which could be judged as COVID-19 negative.
4.If no typical S-type amplification curve(No Ct value) or Ct
value>38 is detected in
the FAM, HEX, and Cy 5 channels of the test sample, it means that
there is a
problem with the quality of the sample or a problem with the
operation. If the result
is invalid, you should find and eliminate the cause, collect the
sample again,
and repeat the test(if the test result is still invalid, please
contact the company) .
【Limitations of Detection Method】
1.Test results of the diagnostic kit can be used only for clinic
reference. The clinical
diagnosis and treatment of patients should be considered in
conjunction with their
symptoms, signs, medical history and other related conditions.
2.False negative results may occur when the concentration of the
detected nucleic
acid in the test sample is below the minimum detection limit of
this kit.
3.Improper handling of the tested sample during collection,
transportation,
storage, and processing can easily result in RNA degradation and
false negative
results.
4.When samples are cross-contaminated during collection,
transportation, storage,
and processing, it is easy to get false positive results.
【Product Performance Index】
1.LOD:The limit of detection is 200 copies/ml.
2.Precision:Coefficient of variation (CV%] of precision Ct value
within batch≤3%.
3.Specificity:There is no cross reaction between the kit and
positive samples, such
as Human Coronavirus HCoV-NL63, Human Coronavirus HCoV-OC43, SARS
Coronavirus, MERS Coronavirus, Influenza A Virus, Influenza B Virus
Yamagata,
Victoria, H1N1 Influenza Virus, H3N2 Influenza Virus, H5N1
Influenza Virus,
H7N9 Influenza Virus, Respiratory Syncytial Virus A,
Adenovirus(type 2) ,
Adenovirus(type 2) , Mycoplasma Pneumoniae, Chlamydia Pneumoniae,
Pertussis, Streptococcus Pneumoniae, Rhinovirus(Type A) etc.
【Precautions】
1.The entire detection process should be performed strictly in
accordance with the
requirements of this manual in the reagent preparation area, sample
processing area,
and PCR amplification area, and the experimental clothes,
instruments,
and consumables in each area should be used independently and
cannot be mixed.
2.To avoid RNA degradation, the sample processing process should be
operated at
0-4℃, and the test should be performed immediately after the
experiment is
completed. Utensil consumables used in sample processing should be
nuclease-free.
3.Negative and positive controls should be set for each experiment.
4.All reagents in the kit should be fully thawed and mixed at room
temperature and
centrifuged immediately before use.
5.All negative and positive controls in the kit should be
transferred to the sample
preparation area and stored separately before the first use.
6.To prevent fluorescence interference, avoid touching the PCR
reaction tube
directly with bare hands, and avoid any marking on the PCR reaction
tube.
7.The instrument amplification related parameters should be set in
accordance with
the relevant requirements of this manual and different batches of
reagents cannot
be mixed.
8.The product waste during the experiment should be detoxified
before being
discarded.
【What is Nucleic Acid?】
Nucleic acids are essential for all forms of life, and it is found
in all cells. Nucleic acids
come in two natural forms called deoxyribonucleic acid (DNA) and
ribonucleic acid (RNA).
Nucleic acids are made of biopolymers, which are
naturally-occurring, repeated sets of
monomers (making polymers) that then create nucleotides, which form
nucleic acids.
To understand the structure of nucleic acid, it is important to
understand the structure
of the nucleotides that make up nucleic acid.
Nucleic acid is an essential part of all living things and is the
building block for both
DNA and RNA. It is found in all cells and also in some viruses.
Nucleic acids have a
very diverse set of functions, such as cell creation, the storage
and processing of
genetic information, protein building, and the generation of energy
cells.
Although their functions may differ, the structures of DNA and RNA
are very similar,
with only a few fundamental differences in their molecular make-up
differentiating them.
【INDEX OF SYMBOL】
【EXPORTER】
Magnus International Limited
F12, New City International Mansion A, 234 Huapao Ave.
Liuyang, Hunan Province 410300 China
Contact: Goodwellmedical@gmail.com
【MANUFACTURER】
Changsha Renji Medical Equipment Co., Ltd.
No.18 Xiangtai Road, Liuyang Jingkai District,
Changsha City, Hunan Province 410300 China
【AUTHORIZED REPRESENTATIVE】
Lotus NL B.V.
Koningin Julianaplein 10, 1e Verd, 2595AA, The Hague, Netherlands.
【FACTORY VIEW】
【FACTORY WORKSHOP】
【PRODUCT AND STANDARDS】
【CERTIFICATIONS】
【FAQ】
1. Q: How about your healthcare and medical product quality?
A: Quality is our top priority. We have a professional quality
control team to ensure the
quality with a high standard and regulated with the pro
certifications. The QC team is
responsible for quality checking in each process and inspect every
batch of our production
and ship them only after QC approval.
2. Q: What is your order MOQ?
A: Please contact the customer service, and the price is negotiable
for large quantity.
3. Q: How about your product packing for shipment or mailing?
A: We prepared well neutral package for our products. We could also
make OEM package,
OEM/ODM Custom Package/Multi-packing package/Cartons with well
protective wrapping.
4. Q: What level of your product price?
A: We are the factory and direct manufacturer and offer competitive
prices. We also offer
the volume pricing with more benefits depend on your order quantity
level. Please let us know
your ordering volume while you make the inquiry to us to help you
obtaining better benefits.
5. Q: What's the price terms?
A: The price is based on FOB of Hunan, China with exchange rate at
RMB6.40 for USD$1.00.
If the exchange rate floating sharply lower than 6.3 or higher than
6.5, we should adjust the
price with real-time exchange rate.
6. Q: What's the payment terms?
A: We accept T/T wire transferring, PayPal, Western Union, etc. 50%
down payment after
signing the contract and rest 50% before shipping confirmation.
7. Q: How could I get some samples?
A: We are able to deduct your samples expense once your orders
confirmed with us.
The shipping fee will be calculated with the sample volume what you
demand.
8. Q: Could you accept OEM order?
A: Yes, we could. We have professional designers who can design the
logo or artwork and offer
best solution & styles for your OEM product choices.
9. Q: How long will the shipping or express take?
A: (1) For the international expresses such as DHL, UPS, FedEx,
TNT, or EMS, etc., it usually
takes 5-7 working days to your location or destination.
(2) If shipping with containers, they will take about 30-60 days to
process depending on
the vary ports or door locations.
(3) We will arrange your products delivery or shipping within 1-3
days after full payment
has been confirmed. We may not ship your products out until your
payments arrive to
our bank account successfully.
10. Q: How do you deliver the goods to us?
A: If the order of small volume package, we could deliver by
courier such as DHL, UPS, FedEx,
TNT, EMS. For the large volume order, we could ship with bulk cargo
containers by sea.
We will provide you cost-saving recommendations for your product
shipping.
11. Q: What is the warranty time.
A: One year f each product.
【SHIPPING】
We are the trusted healthcare and medical supplies from China.
Be free to contact us at any time!
You are very welcome to email us today at:
Goodwellmedical@gmail.com for the inquiry!
We are excited to build a reliable and long-term business with you
at our best!