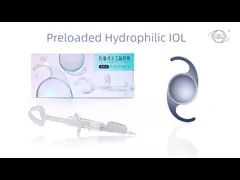
Product Details
Modified C Haptic Hydrophilic Intraocular Lens In Cataract
Surgeries
Hydrophobic acrylic (HA) materials are currently the ‘gold
standard’ material for intraocular lenses used in cataract surgery.
However, hydrophobicity is a broad classification that may be
defined based on surface and bulk material properties.
Product Features
- Top material: high purity Hydrophilic Acrylic from Contamac UK with
excellent material uniformity, good biocompatibility, smooth
surface, low silicone oil adsorption rate, bacteria and other
sediments are not easy to adhere, no calcification and glistening.
- Zero spherical aberration: provides patients with improved contrast
sensitivity in mesopic, photopic and dim light conditions.
- Excellent Stability: Medified C loop Provides excellent stability
for the intraocular lens in the capsular bag.
Product Parameters
| Product | Aspherical Hydrophilic Acrylic Intraocular Lens |
| Manufacturer | Henan Universe IOL R&M Co., Ltd |
| Model | PCF60/A |
| Overall Length (mm) | 12.5 |
| Optic diameter (mm) | 6.0 |
| Material | Hydrophilic Acrylic |
| Optic Type | Biconvex |
| Haptic anglulation | 5° |
| Haptic Type | Modified “C” Loop |
| Edge Type | 360° square edge |
Aspherical Hydrophilic Acrylic Intraocular Lens have higher water
content and a lower refractive index relative to hydrophobic
acrylic IOLs, minimizing glare, external and internal reflections,
and other unwanted visual phenomena.
Company Profile
Henan Universe Intraocular Lens Research and Manufacture Co., Ltd.
was founded in 1991, is one of the first enterprises in China to
produce intraocular lenses and related products. It has introduced
advanced American technology and established a complete automated
production line, integrating R&D, production, and sales. After
30 years of development, it has become China A benchmark enterprise
in the field of intraocular lenses.
Till now, our company has products in sale, such as PMMA
Intraocular Lens, Hydrophilic Acrylic Intraocular Lens, Glaucoma
Drainage Implants, Hydroxypropyl Methylcellulose, Sodium
Hyaluronate, Surgical Suture Needles with Thread, Disposal Injector
for Foldable IOL and so on. Over 2 million Universe IOLs has been sold since their launch in
1991, with very low incidences of adverse effects and serious
adverse events.
The highest eminence is to be gianed step by step. Henan Universe
Intraocular Lens Research and Manufacture Co., Ltd. will work hard
and innovate constantly to improve the quantity of products,
elevate the core competitiveness of the enterprise and make even
greater contribution to the human health undertaking.