
Add to Cart
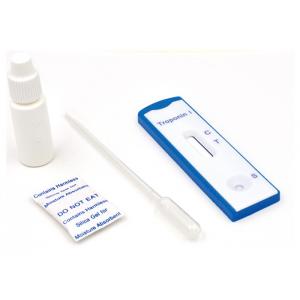
High Sensitivity Troponin I cardiac blood test, professional use, 4mm cassette, cut-off 1ng/ml
Intended Use:
The Troponin I Rapid Test Device (Whole Blood/Serum/Plasma) is a rapid, two-site sandwich immunoassay for the detection of human cardiac Troponin I (cTnI) levels in human whole blood.
Summary:
Discovered by Ebashi, Troponins are regulatory proteins in cardiac muscle that modulate the interaction between actin and myosin, during the calcium-mediated contraction of cardiac muscle. Three distinct tissue specific isoforms of Troponin I have been identified, two in skeletal muscle and one in cardiac muscle. The cardiac isoform of Troponin I (cTnI) has an additional sequence of 31 amino acids at the N terminal end that accounts for cardiac specificity, with a molecular weight of 22.5 kDa. This absolute specificity of Troponin I for cardiac tissue makes it an ideal biomarker for myocardial injury. Clinical study results have demonstrated that elevated levels of cardiac Troponin I (cTnI) are detectable in blood stream within 4 to 6 hours after the onset of chest pain, reach peak concentration in approximately 12 hours and remain elevated for 3-10 days following acute myocardial infarction. Thus cardiac Troponin I (cTnI) meets all the criterion laid down by National Academy of Clinical Biochemistry (NACB) for an ideal cardiac biomarker in early identification and risk stratification of patients with chest pain suggestive of ischaemia and identification of patients that present after infarction.
Test Principle:
The Troponin I Rapid Test Device utilizes the principle of immunochromatography, with a unique two-site sandwich immunoassay on a nitrocellulose membrane. The conjugate pad contains two components-monoclonal anti-cTnI conjugated to colloidal gold and rabbit IgG conjugated to colloidal gold. As the test sample flows through the membrane assembly of the device, the highly specific anti-cTnI antibody - colloidal gold conjugate complexes with cTnI in the sample and travels on the membrane due to capillary action along with rabbit IgG-colloidal gold conjugate. This sample moves further on the membrane to the test region (T) where it is immobilized by another specific anti-cTnI antibody coated on the membrane leading to the formation of a pink-purple band. A detectable coloured band is formed if cTnI level is equal to or greater than 0.3 ng/ml. The absence of this coloured band in the test region indicates cTnI concentration < 0.3 ng/ml. The unreacted conjugate along with unbound complex if any, move further on the membrane and are subsequently immobilized by the anti-rabbit antibodies coated on the membrane at the control region (C) forming a pink-purple coloured band. The control band formation is based on the ‘Rabbit / anti-Rabbit globulin’ system. Since it is completely independent of the analyte detection system, it facilitates formation of consistent control band signal independent of the analyte concentration. This control band acts as a procedural control and serves to validate test results.
SPECIMEN COLLECTION AND PREPARATION
INTERPRETATION OF RESULTS
POSITIVE:* Two lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in the test line region (T).
*NOTE: The intensity of the color in the test line region (T) will vary depending on the concentration of TP antibodies present in the specimen. Therefore, any shade of color in the test line region (T) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No line appears in the test line region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
PERFORMANCE CHARACTERS:
Sensitivity and Specificity
The Troponin I Rapid Test Device (Whole Blood/Serum/Plasma) has been evaluated with a leading commercial cTnI EIA test using clinical specimens. The results show that the sensitivity of the Troponin I Rapid Test Device (Whole Blood/Serum/Plasma) is 98.3% and the specificity is 98.4% relative to the leading EIA test.
Troponin I Rapid Test Device vs. EIA
| Method | EIA Test | Total Results | ||
| Troponin I Rapid Test Device | Results | Positive | Negative | |
| Positive | 113 | 6 | 119 | |
| Negative | 2 | 493 | 495 | |
| Total Results | 115 | 499 | 614 | |
Relative Sensitivity: 98.3%(93.9%-99.8%)*
Relative Specificity: 98.4%(97.4%-99.6%)*
Accuracy: 98.7%(97.5%-99.4%)*
*95% Confidence Interval
| ORIENT NEW LIFE MEDICAL CO., LTD. | |
| Contact: | Jerry Meng |
| Email: | Jerry @ newlifebiotest .com |
| Tel. | +86 18657312116 |
| SKYPE | enetjerry |
