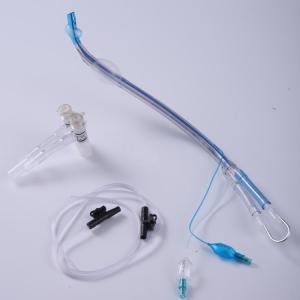
Product Details
Pre-Loaded Stylet PVC Endobronchial Tube Double Balloon
Endobronchial Tubes
| Product name | PVC Endobronchial Tube |
| Size ID(Fr) | Left:28Fr,32Fr,35Fr,37Fr,39Fr,41Fr;Right:28Fr,32Fr,35Fr,37Fr,39Fr,41Fr; |
| Material | Medical Grade PVC |
| Type | Surgical Supplies |
| Certificate | CE&ISO13485 |
| Application | Surgical Operation |
| MOQ | 500 pcs/Per size |
| Color | Transparent |
| Feature | Separation of Lungs |
| Sterile | EO gas sterile |
| Service | OEM/ODM |
| Port | FOB Guangzhou or Shanghai |
| Sample Time | 4-7working days |
| Payment method | T/T |
| Shipment | Express,Air ,Sea |
Endobronchial tube is used for one-lung ventilation Made of PVC in
medical grade. Available with Left-sided and Right-sided types.The
cuffs, two lumens and pilot balloons are imprinted with Tracheal (clear) or Bronchial (blue) for easy
identification. Smooth inner lumens allow easy passage of
bronchoscopes. Pre-loaded with stylet to help tube maintain shape
for easier intubation. Packed with switch connector and two Low
Friction suction catheters.
Product Detail:
Company Profile
Guangzhou ORCL Medical Co., Ltd Founded in 2010, it is located in Nansha, the national free trade
zone, with a plant area of 6000 square meters and a purification
workshop area of 3300 square meters in line with GMP standards. It
is mainly engaged in the research and development, production and
sales of medical catheters in the fields of " Ureteral
catheter,anesthesia and respiration". The products are widely used
in clinical medical fields such as surgery, treatment, medical aid
and nursing care; Company approval TUV:ISO13485 , TUV:ISO11135 ,
GMP certification, national high-tech enterprise certification,
intellectual property standards implementation certification, etc.,
has obtained CFDA registration certification, EU TUV:CE (0123)
certification, quality assurance.
The company has R & D technology platform in professional fields,
focusing on product innovation, service patent cooperation,
industry university research cooperation and medical product
project cooperation; it has obtained domestic registration
certificate: ordinary endotracheal intubation, enhanced
endotracheal intubation, PVC laryngeal mask, silicon laryngeal
mask, double lumen air tube intubation, silicon catheter, silicon
temperature measuring catheter, catheterization bag and atomizer;
OEM and ODM manufacturing: medical guide wire, medical spring,
medical one-way valve, balloon catheter, medical plastic parts
molding, medical silicon parts injection, medical mold, etc.